John F.Salmon - Kanski's Clinical Ophthalmology
Glaucoma is the term that is used to describe a group of conditions that have in common a chronic progressive optic neuropathy that results in characteristic morphological changes at the optic nerve head and in the retinal nerve fibre layer. Progressive retinal ganglion cell death and visual field loss are associated with these changes. Intraocular pressure is a key modifiable factor.
Glaucoma is characterized by the three main characteristics (Grefe Triad):
- High intraocular pressure
- Decrease in field of vision
- Damage of the optic nerve
Intraocular pressure (IOP) is determined by the balance between the rate of aqueous production and the rate of aqueous outflow. The latter is related to factors that include the resistance encountered in the trabeculum and the level of episcleral venous pressure.
Aqueous humour is produced from plasma by the ciliary epithelium of the ciliary body pars plicata, using a combination of active and passive secretion. A high-protein filtrate passes out of fenestrated capillaries (ultrafiltration) into the stroma of the ciliary processes, from which active transport of solutes occurs across the dual-layered ciliary epithelium. The osmotic gradient thereby established facilitates the passive flow of water into the posterior chamber.
Secretion is subject to the influence of the sympathetic nervous system, with opposing actions mediated by beta-2 receptors (increased secretion) and alpha-2 receptors (decreased secretion). Enzymatic action is also critical – carbonic anhydrase is among those playing a key role.
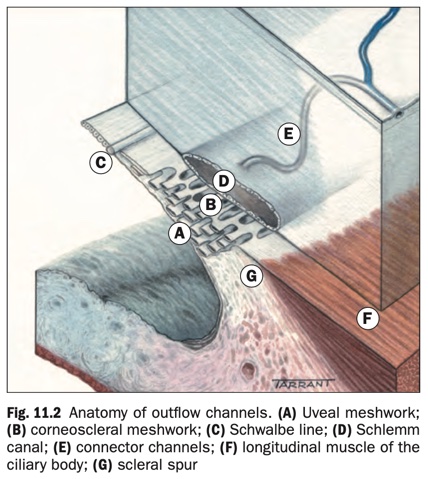
- The trabecular meshwork (trabeculum) is a sieve-like structure at the angle of the anterior chamber (AC) through which 90% of aqueous humour leaves the eye. It has three components (Fig. 11.2):
- The uveal meshwork is the innermost portion, consisting of cord-like endothelial cell-covered strands arising from the iris and ciliary body stroma. The intertrabecular spaces are relatively large and offer little resistance to the passage of aqueous.
- The corneoscleral meshwork lies external to the uveal meshwork to form the thickest portion of the trabeculum. It is composed of layers of connective tissue strands with overlying endothelial-like cells. The intertrabecular spaces are smaller than those of the uveal meshwork, conferring greater resistance to flow.
- The juxtacanalicular (cribriform) meshwork is the outer part of the trabeculum and links the corneoscleral meshwork with the endothelium of the inner wall of the canal of Schlemm. It consists of cells embedded in a dense extracellular matrix with narrow intercellular spaces and offers the major proportion of normal resistance to aqueous outflow.
- The Schlemm canal is a circumferential channel within the perilimbal sclera. The inner wall is lined by irregular spindle-shaped endothelial cells containing infoldings (giant vacuoles) that are thought to convey aqueous via the formation of transcellular pores. The outer wall is lined by smooth flat cells and contains the openings of collector channels, which leave the canal at oblique angles and connect directly or indirectly with episcleral veins. Septa commonly divide the lumen into 2–4 channels.
Aqueous flows from the posterior chamber via the pupil into the AC, from where it exits the eye via three routes (Fig. 11.3).
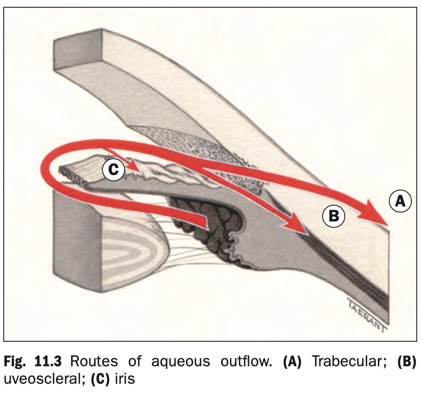
- Trabecular outflow (90%): aqueous flows through the trabeculum into the Schlemm canal and then the episcleral veins. This is a bulk flow pressure-sensitive route so that increasing IOP will increase outflow.
- Uveoscleral drainage (10%): aqueous passes across the face of the ciliary body into the suprachoroidal space and is drained by the venous circulation in the ciliary body, choroid and sclera.
- Iris: some aqueous also drains via the iris.
Glaucoma may be congenital (developmental) or acquired. Open-angle and angle-closure types are distinguished based on the mechanism by which aqueous outflow is impaired with respect to the AC angle configuration. Distinction is also made between primary and secondary glaucoma. In the latter a recognizable ocular or non-ocular disorder contributes to elevation of IOP.
Primary open-angle glaucoma (POAG) is a chronic, progressive optic neuropathy of adult onset. It is characterized by:
- Retinal nerve fibre layer thinning.
- Glaucomatous optic nerve damage.
- Characteristic visual field loss as damage progresses.
- An open anterior chamber angle.
- Absence of signs of secondary glaucoma or a non-glaucomatous
cause for the optic neuropathy.
- IOP is a key modifiable risk factor.
POAG is the most prevalent type of glaucoma in people of European and African ethnic origin
Pathogenesis of glaucomatous optic neuropathy
Retinal ganglion cell death in glaucoma occurs predominantly through apoptosis (programmed cell death) rather than necrosis. The preterminal event is calcium ion influx into the cell body and an increase in intracellular nitric oxide. Glutamine metabolism is intrinsically involved. After initial injury, a cascade of events results in astrocyte and glial cell proliferation and alterations in the extracellular matrix of the lamina cribrosa, with subsequent optic nerve head remodelling.
The process of glaucomatous damage and the relationship with IOP and other potential influences is still poorly understood. One or both of the following mechanisms may be involved:
Direct mechanical damage to retinal nerve fibres at the optic nerve head, perhaps as they pass through the lamina cribrosa. Accumulating evidence of the influence of mechanical deformability in the region of the lamina cribrosa supports this.
Ischaemic damage, possibly due to compression of blood vessels supplying the optic nerve head. This may relate to ocular perfusion pressure as a possible risk factor for glaucoma.
Common pathways of damage. Both mechanisms might lead to a reduction in axoplasmic flow, interference with the delivery of nutrients or removal of metabolic products, deprivation of neuronal growth factors, oxidative injury and the initiation of immune-mediated damage.
Risk factors
IOP.
The higher the IOP, the greater the likelihood of glaucoma. Asymmetry of IOP of 4 mmHg or more is also significant.
Age.
POAG is more common in older individuals.
Race.
It is significantly (perhaps four times) more common, develops at an earlier age and may be more difficult to control
in black individuals than in whites.
Family history of POAG.
First-degree relatives of patients with
POAG are at increased risk. An approximate risk to siblings is four times and to offspring twice the normal population risk, though surveyed figures vary.
Diabetes mellitus.
Longitudinal studies show no increased risk of glaucoma. Selection bias probably explains why clinic- based studies report a higher prevalence of glaucoma in people with diabetes.
Myopia is associated with an increased incidence of POAG and myopic eyes may be more susceptible to glaucomatous damage.
Anti-VEGF (vascular endothelial growth factor) therapy.
Patients undergoing anti-VEGF therapy for age-related macular degeneration or diabetic macular oedema are at risk of sustained IOP elevation. This is more likely to occur after recurrent injections with bevacizumab than with ranibizumab. The risk is significantly greater for patients with glaucoma than for normal individuals. The risk that glaucoma surgery will be needed increases after six injections.
Contraceptive pill.
Recent research suggests that long-term use of the oral contraceptive pill may increase the risk of glaucoma, perhaps by blocking a protective oestrogen effect.
Vascular disease. A range of systemic conditions linked to vascular compromise may be associated, though clear-cut relationships have proved difficult to demonstrate consistently. Systemic hypertension, cardiovascular disease, diabetes and vasospastic conditions such as migraine have all been implicated. Poor ocular perfusion may be a risk factor for glaucoma progression.
Translaminar pressure gradient. Studies suggest that a difference in the levels of IOP and orbital CSF pressure may increase the likelihood of the development and progression of glaucomatous damage, perhaps due to associated deformation of the lamina cribrosa.
Optic disc area. Large discs are more vulnerable to damage.
Ocular perfusion pressure is the difference between the arterial BP and the IOP and has been shown in population studies to be linked to increased risk for the development and progression of glaucoma.
Diagnosis
History
Visual symptoms will usually be absent, unless damage is advanced. Sometimes symptomatic central field defects may occur at an early stage, in the presence of a relatively normal peripheral field.
Previous ophthalmic history
Specific enquiry should be made about:
- Refractive status, as myopia carries an increased risk of POAG and hypermetropia of primary angle-closure glaucoma (PACG).
- Causes of secondary glaucoma such as ocular trauma or inflammation. Previous eye surgery, including refractive surgery which may affect IOP readings.
Family history
- POAG or related conditions such as OHT.
- Other ocular disease in family members.
Past medical history
- Asthma, heart failure or block, peripheral vascular disease,
which are contraindications to the use of beta-blockers.
- Head injury, intracranial pathology including stroke as these conditions may cause optic atrophy or visual field
defects.
- Vasospasm: migraine and Raynaud phenomenon.
- Diabetes, systemic hypertension and cardiovascular
disease may increase the risk of POAG.
- Oral contraceptive pill for several years may be associated
with an increased risk of glaucoma.
Current medication
- Steroids including skin cream and inhalants.
- Oral beta-blockers may lower IOP.
- Social history including smoking and alcohol intake, especially if toxic/nutritional optic neuropathy is suspected.
- Allergies, particularly to any drugs likely to be used in glaucoma treatment, e.g. acetazolamide is contraindicated
if there is a history of sulfonamide allergy.
Examination
- Visual acuity (VA) is likely to be normal except in advanced glaucoma.
- Pupils. Exclude a relative afferent pupillary defect (RAPD). If initially absent but develops later, this constitutes an indicator of substantial progression.
- Colour vision assessment such as Ishihara chart testing if there is any suggestion of an optic neuropathy other than glaucoma.
- Slit lamp examination. Exclude features of secondary glaucoma such as pigmentary and pseudoexfoliation.
- Tonometry prior to pachymetry, noting the time of day.
- Gonioscopy.
- Optic disc examination for glaucomatous changes should be performed with the pupils dilated, provided gonios- copy does not show critically narrow angles. Red-free light can be used to detect RNFL defects.
Management
The primary aim of treatment is to prevent functional impairment of vision within the patient’s lifetime, by slowing the rate of ganglion cell loss. Currently the only proven method of achieving this is to reduce IOP. Both higher mean IOP and substantial variation in IOP are predictive of progressive visual field loss in patients with glaucoma, whether newly diagnosed or advanced.
- Medical therapy
- Laser trabeculoplasty
- Surgery (Trabeculectomy)
The term ‘angle closure’ refers to occlusion of the trabecular meshwork by the peripheral iris (iridotrabecular contact – ITC), obstructing aqueous outflow. Angle closure can be primary, when it occurs in an anatomically predisposed eye, or secondary to another ocular or systemic factor. PACG may be responsible for up to half of all cases of glaucoma globally and is particularly common in Asia. It progresses rapidly and is more likely to result in visual loss than POAG.
Mechanism
The mechanisms involved in angle closure can be categorized according to the anatomical level (anterior to posterior) at which causative forces act. In many patients more than one level is contributory.
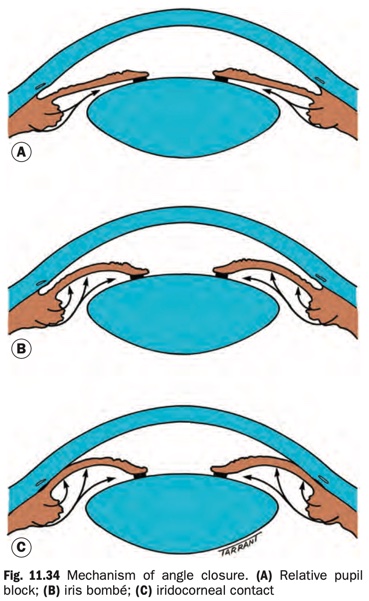
Relative pupillary block
- Failure of physiological aqueous flow through the pupil
leads to a pressure differential between the anterior and posterior chambers, with resultant anterior bowing of the iris (Fig. 11.34).
- Usually anatomically relieved by peripheral iridotomy, which equalizes anterior and posterior chamber pressure. Reduction of IOP will follow provided the angle has opened adequately. This may not occur if there are substantial PAS or an additional mechanism of angle closure is in effect. Trabecular meshwork damage can prevent normalization of IOP even with an anatomically open angle.
- The lens vault quantifies the portion of the lens located anterior to the anterior chamber angle. A common definition is the distance between the anterior pole of the lens and a horizontal line joining the scleral spur at diametrically opposite locations. A large lens vault is independently associated with angle closure, though it is not clear whether this is entirely via a pupillary block or non-pupillary block mechanism, or both.
Non-pupillary block
- Thought to be important in many Asian patients.
- Associated with a deeper AC than found in those with pure
pupillary block.
- Patients with non-pupillary block, particularly those with
plateau iris, tend to be younger than those with pure
pupillary block.
- An element of pupillary block is invariably present,
but angle closure is not fully relieved by iridotomy. The term ‘mixed mechanism’ has been suggested to describe glaucoma in which both significant pupillary block and non-pupillary block mechanisms co-exist.
Lens-induced angle closure.
Angle closure that is predominantly lens-induced or due to a retrolenticular cause is often categorized as secondary (see below).
- This includes those cases in which a sudden change in
lens volume and/or position leads to an acute or subacute
IOP rise.
- Usually rapid progression of lens intumescence (phacomorphic glaucoma) or anterior lens subluxation.
- All cases of pupillary block have a phacomorphic element
that increases with age as the lens thickens.
Retrolenticular
- Malignant glaucoma (‘ciliolenticular block’).
- Posterior segment causes of secondary angle closure (see
below).
"Combined mechanism" has been proposed as a formal
label for the combination of angle-closure and open-angle
elements.
Reduced aqueous outflow in angle closure has been postulated
to be caused by the following mechanisms in varying degree:
- Appositional obstruction by the iris.
- Degeneration of the TM itself due to chronic or intermittent contact with the iris or damage sustained due to
elevated IOP.
- Permanent occlusion of the TM by PAS. The prognosis for
IOP control correlates well with the extent of PAS.
Risk factors
- Age. The average age of relative pupillary block is about 62 years at presentation. Non-pupillary block forms of primary angle closure tend to occur at a younger age.
- Gender. Females are more commonly affected than males.
- Race. Particularly prevalent in Far Eastern and Indian Asians, where non-pupillary block is relatively more significant.
- Family history. Genetic factors are important but poorly defined, with an increased prevalence of angle closure in
family members.
- Refraction. Eyes with ‘pure’ pupillary block are usually hypermetropic. Non-pupillary block mechanism can occasionally occur in myopic eyes. Up to one in six patients with hypermetropia of one dioptre or more are primary angle closure suspects, so routine gonioscopy should be considered in all adult who are hypermetropic.
- Axial length. Short eyes tend to have a shallow AC secondary to a relatively anterior lens position. Eyes with nanophthalmos (axial length less than 20 mm) have a very short eye and are at particular risk.
Diagnosis
Symptoms:
- Precipitating factors include watching television in a darkened room, pharmacological mydriasis or rarely miosis, adoption of a semi-prone position (e.g. reading), acute emotional stress and occasionally systemic medication: parasympathetic antagonists or sympathetic agonists including inhalers, motion sickness patches and cold/flu remedies (mydriatic effect), topiramate and other sulfa derivatives (ciliary body effusion).
- Presentation can be with intermittent symptoms of blurring (‘smoke-filled room’) and haloes (‘rainbow around lights’) due to corneal epithelial oedema, or acutely with markedly decreased vision, redness and ocular/periocular pain and headache. Abdominal pain and other gastrointestinal symptoms may occur.
- Most patients with angle closure are asymptomatic, including most of those with intermittently or chronically elevated IOP.
Signs:
Acute primary angle closure (APAC), previously called ‘acute glaucoma’
- VA is usually 6/60 to HM.
- The IOP is usually very high (50–80 mmHg).
- Conjunctival hyperaemia with violaceous circumcorneal injection.
- Corneal epithelial oedema (Fig. 11.36A).
- The AC is shallow and aqueous flare is usually present.
- A non-reactive mid-dilated vertically oval pupil is classic
(Fig. 11.36B).
- The fellow eye typically shows an occludable angle. If this
is not present, secondary causes of angle closure should be
considered.
Resolved APAC
- Early: low IOP (ciliary body shutdown and effect of intensive treatment), folds in Descemet membrane if IOP has reduced rapidly (Fig. 11.37A), optic nerve head congestion, choroidal folds.
- Late: iris atrophy with a spiral-like configuration, glaukomflecken (Fig. 11.37B) (white foci of necrosis in the superficial lens) and other forms of cataract and irregular pupil due to iris sphincter/dilator damage and posterior synechiae (Fig. 11.37C). The optic nerve may be normal or exhibit varying signs of damage, including pallor and/or cupping (Fig. 11.37D).
- The greater the duration of an attack of APAC and the extent of post-APAC PAS, the lower the likelihood of IOP control with medical treatment alone.
Subacute angle closure is used to describe the clinical scenario of intermittent episodes of spontaneously resolving mild/ moderate APAC, usually in patients with pupillary block. The clinical course may be chronic, or may culminate in a more severe/unresolving episode of APAC.
Chronic presentation
- VA is normal unless damage is advanced.
- The AC is usually shallower in relative pupillary block than
non-pupillary block.
- IOP elevation may be only intermittent.
- ‘Creeping’ angle closure is characterized by a gradual
band-like anterior advance of the apparent insertion of the iris. It starts in the deepest part of the angle superiorly and spreads circumferentially.
- Intermittent ITC may be associated with the formation of discrete PAS, individual lesions having a pyramidal (‘sawtooth’) appearance (see Fig. 11.33B).
- Optic nerve signs depend on the severity of damage.
(p. 376)
Investigation
- Anterior segment OCT (AS-OCT – see Fig. 11.16, ultrasound biomicroscopy) or Scheimpflug photography may be useful to supplement gonioscopic findings and for patient education.
- Anterior chamber depth measurement is helpful in some cases.
- Biometry if lens extraction is considered.
- Posterior segment ultrasonography in atypical cases to
exclude causes of secondary angle closure.
- Provocative testing. This may aid decision-making in some
circumstances, particularly when plateau iris syndrome is suspected.
- Pharmacological mydriasis is a poor discriminator and
carries a small risk of precipitating APAC in susceptible
patients without a patent iridotomy.
- Dark room/prone provocative test (DRPPT): the patient
sits in a dark room, face down for 1 hour without sleeping (sleep induces miosis). The IOP is checked before and immediately after the test, as IOP can normalize very rapidly. An IOP rise of 8 mmHg or more is considered significant. Gonioscopy without indentation should be used to confirm closure of the angle. If the test is positive in a patient with a patent laser iridotomy, the underlying anatomical cause is usually plateau iris, which can be confirmed with an OCT scan. A positive response is abolished after lens extraction.
Treatment
APAC:
Initial treatment
- The patient should lie down in a supine position to
encourage the lens to shift posteriorly under the influence
of gravity.
- cetazolamide 500mg is given intravenously if IOP >50mmHg and orally (not slow-release) if IOP is <50 mmHg.
- Contraindications include sulfonamide allergy and angle
closure secondary to topiramate or other sulfonamide
derivatives.
- A single dose of apraclonidine 0.5% or 1%, timolol 0.5%
and prednisolone 1% or dexamethasone 0.1% is instilled
into the affected eye, leaving 3–5 minutes between each.
- Pilocarpine 2% one drop to the affected eye, repeated after half an hour and one drop of 1% into the fellow eye. This should not be repeated if the IOP remains >40 mmHg as ischaemia may compromise its action, may exert a forward vector and excessive dosing carries a systemic
toxicity risk.
- Analgesia and an antiemetic may be required.
Resistant cases
- Central corneal indentation with a squint hook or indentation goniolens to force aqueous into the angle. Epithelial oedema can be cleared first with topical 50% glycerol to improve visualization and to avoid abrasion.
- Mannitol 20% 1–2 g/kg intravenously over 1-hour, oral glycerol 50% 1 g/kg, or oral isosorbide 1–1.5 g/kg, having checked for contraindications.
- Early laser iridotomy or iridoplasty after clearing corneal oedema with glycerol.
- Paracentesis is effective, but carries a small risk of lens damage.
- Surgical options: peripheral iridectomy, lens extraction, goniosynechialysis, trabeculectomy and cyclodiode laser treatment.
Subsequent medical treatment
- Pilocarpine 2% four times daily to the affected eye and 1%
four times daily to the fellow eye.
- Topical steroid (prednisolone 1% or dexamethasone 0.1%)
four times daily if the eye is acutely inflamed.
- Any or all of the following should be continued according to response: timolol 0.5% twice daily, apraclonidine 1% three times daily and oral acetazolamide 250 mg four times
daily.
Bilateral laser iridotomy is performed once an attack has been
broken, signified by a clear cornea and preferably normal IOP.
Topical steroids are continued for at least a week.
Gonioscopy needs to be repeated to ensure that the angle is
open.
Subsequent management is as for post-iridotomy chronic
PAC/PACG. A low threshold may be adopted for cataract surgery, particularly if a significant phacomorphic element is suspected. Trabeculectomy is occasionally necessary for persistent IOP elevation despite a successfully opened angle.
PACS:
- Laser iridotomy (Fig. 11.38). The ZAP trial concludes that laser PI has a modest prophylactic effect, but should only be offered to those with the highest risk of developing PACG.
- If significant ITC persists after iridotomy, options include observation (most), laser iridoplasty and long-term pilocarpine prophylaxis, e.g. 1% twice daily. If symptomatic cataract is present, lens extraction usually opens the angle (Fig. 11.39). If IOP is elevated, then by definition PAC is present.
PAC and PACG:
- Management is the same as that recommended for PACS, but with a lower threshold for further intervention if angle widening is inadequate after iridotomy, particularly if IOP remains elevated.
- Review. Urgency and intensity of treatment and frequency of review is tailored to the individual patient, considering IOP, extent of angle closure and glaucomatous damage, if present.
- Medical treatment as for POAG may be required for eyes with substantial synechial closure or with persistently elevated IOP despite an opened angle.
- Trabeculectomy with mitomycin C is an option, but may result in malignant glaucoma.
- Phacoemulsification with intraocular lens implantation is highly effective as it corrects hypermetropia, deepens the anterior chamber and opens the filtration angle. IOP control is achieved in almost all patients whose IOP is within the normal range prior to surgery and in up to 80% of those whose IOP is higher than normal preoperatively. The EAGLE study (Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma) concludes that clear lens extraction with IOL implantation shows greater efficacy and is more cost-effective than laser peripheral iridotomy in patients with primary angle closure and IOP >29 mmHg or in patients with primary angle-closure glaucoma.
Secondary open-angle glaucoma can be subdivided on the basis of the site of aqueous outflow obstruction.
- Pre-trabecular, in which aqueous outflow is obstructed by a
membrane covering the trabeculum (Fig. 11.40A), which may consist of:
- Fibrovascular tissue (neovascular glaucoma).
- Endothelial cellular membranous proliferation (iridocorneal endothelial syndrome).
- Epithelial cellular membranous proliferation (epithelial
ingrowth).
- Trabecular, in which the obstruction occurs as a result of
‘clogging up’ of the meshwork (Fig. 11.40B) and secondary degenerative changes.
- Pigment particles (pigmentary glaucoma).
- Red blood cells (red cell glaucoma).
- Degenerate red cells (ghost cell glaucoma).
- Macrophages and lens proteins (phacolytic glaucoma).
- Proteins (probably an element in hypertensive uveitis).
- Pseudoexfoliative material (pseudoexfoliation glaucoma).
- Trabecular glaucoma may also be caused by alteration of the trabecular fibres themselves by oedema (e.g. trabeculi- tis in hypertensive uveitis) or scarring (e.g. post-traumatic angle recession).
- Post-trabecular in which the trabeculum itself is normal but aqueous outflow is impaired as a result of elevated episcleral venous pressure.
- Carotid-cavernous fistula.
- Sturge–Weber syndrome.
- Obstruction of the superior vena cava.
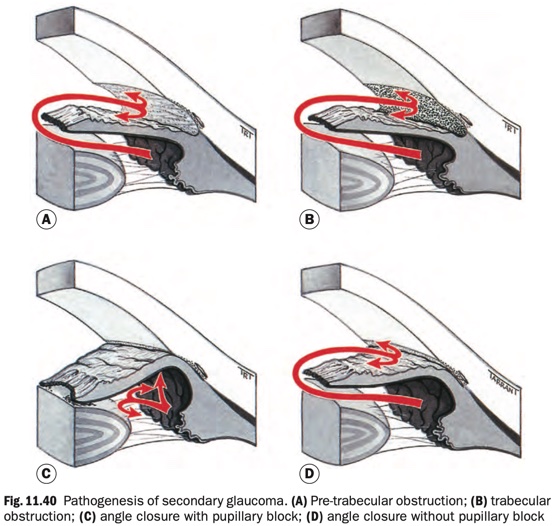
- With pupillary block (Fig. 11.40C)
- Seclusio pupillae (360° posterior synechiae), usually secondary to recurrent iridocyclitis.
- Subluxated lens.
- Phacomorphic glaucoma.
- Capsular block syndrome with 360° iris–capsule adhesion
in a pseudophakic eye.
- Aphakic pupillary block.
- Anterior chamber lens implant without a patent iridotomy.
- Without pupillary block (Fig. 11.40D)
- Secondary causes of PAS such as advanced neovascular
glaucoma and chronic anterior uveitis.
- Cilio-choroidal effusion.
- Capsular block syndrome without iris–capsule adhesion.
- Ciliary body/iris cyst or other ciliary body or posterior
segment tumour.
- Contraction of retrolenticular fibrovascular tissue such
as in proliferative vitreoretinopathy and retinopathy of
prematurity.
- Malignant glaucoma (ciliolenticular block).
Thought to be caused by trabeculodysgenesis.
Presentation usually occurs when an abnormality such as corneal haze, large (buphthalmos) or asymmetrical eyes, watering, photophobia or blepharospasm is noticed by parents or a health professional
- Pigmentary Glaucoma
- Neovascular Glaucoma
- Inflammatory Glaucoma
- Steroid-induced Glaucoma
- Traumatic Glaucoma
- Prostaglandin derivatives (enhancement of uveoscleral aqueous outflow, drug name suffix: -oprost)
- Beta-blockers (reduce IOP by decreasing aqueous production, mediated by an effect on the ciliary epithelium, drug name suffix: -olol)
- Alpha-2 agonists (decreases aqueous synthesis via an effect on the ciliary epithelium and increases uveoscleral outflow, drug name suffix: -onidine)
- Topical carbonic anhydrase inhibitors (CAI, inhibits aqueous secretion, they are chemical relatives to sulfonamide antibiotics)
- Miotics (cholinergic agonists, predominantly used in the treatment of angle closure, also reduce IOP by contraction of the ciliary muscle, which increases the facility of aqueous outflow through the trabecular meshwork)
- Relieve pupil block
- Lower IOP: topical agents. Usually miotics (Pilocarpine, carbachol), acetazolamide (Diacarb - CAI, diuretic)
- Reduce pain: analgesics
- Reduce nausea and vomiting: anti-emetics
- Laser treatment (trabeculoplasty, iridotomy, iridoplasty)
- Trabeculotomy
- Non-penetrating Glaucoma Surgery
- Minimally Invasive Glaucoma Surgery
- Drainage Shunts



